Are you curious about the properties of esters? Do you want to know whether esters have a leaving group, and if so, how to make them one? Perhaps you’re wondering whether ether can act as a leaving group, or whether esters are electron donating or withdrawing. In this blog post, we’ll explore the characteristics of esters, how they’re named, and what makes a good leaving group. We’ll even delve into the mechanisms of nucleophilic acyl substitution in basic conditions. So grab a cup of coffee and get ready to expand your knowledge on this fascinating topic.
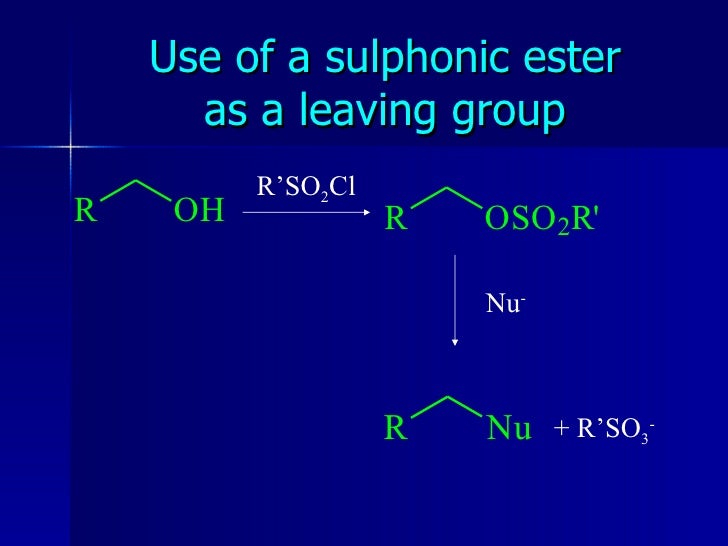
The Function of a Leaving Group in Ester Reactions: Understanding its Role.
Esters are commonly encountered in organic chemistry and play a significant role in various chemical reactions. One of the fundamental questions that arise in the context of esters is whether they have a leaving group or not. The answer is yes, esters have a leaving group, which is the alkoxide group (RO-) attached to the carbonyl carbon.
In basic conditions, the mechanism of a nucleophilic acyl substitution takes place, where the alkoxide leaving group of the ester is replaced by an incoming alkoxide nucleophile, leading to the formation of a different ester. This reaction is essential in various organic synthesis processes, such as the formation of carboxylic acids from esters.
It is crucial to note that the leaving group ability of the ester is dependent on the stability of the alkoxide ion formed after the leaving group departs. Generally, a more stable alkoxide ion will lead to a better leaving group ability for the ester.
Therefore, the characteristics of the ester’s leaving group play a crucial role in determining the reactivity of the ester in various chemical reactions. The leaving group must have certain characteristics, such as being able to stabilize the negative charge that arises after the departure of the leaving group.
In summary, esters do have a leaving group, which is the alkoxide group attached to the carbonyl carbon. The leaving group ability of the ester is dependent on the stability of the alkoxide ion formed after the leaving group departs. Understanding the properties and characteristics of the ester’s leaving group is crucial in determining the reactivity of the ester in various chemical reactions.
>> Must read When should I apply lip oil?
Transforming an Ester into a Leaving Group: A Guide.
Esters are organic compounds that have a carbonyl group bonded to an alkyl group and an alkoxy group. The alkoxy group can act as a leaving group when the ester undergoes a reaction. However, it is necessary to make the ester a leaving group to facilitate its reaction. In acidic conditions, the mechanism of making an ester a leaving group involves protonation of the alcohol reactant.
During the reaction, the alcohol reactant adds to the ester carbonyl, and proton transfer to the ester’s alkoxy group increases its ability to act as a leaving group. This protonation also allows the reforming of the C=O carbonyl bond, which removes the leaving group from the molecule. The subsequent deprotonation by water forms the ester product.
This mechanism is essential in many reactions, including esterification and transesterification reactions. The acidic conditions also help to catalyze the reaction and increase the rate of the reaction.
It is noteworthy that the ester’s ability to act as a leaving group depends on the nature of the alkyl group and the alkoxy group. The ester’s leaving group ability increases with the electron-withdrawing nature of the alkyl group and the electron-donating nature of the alkoxy group.
In summary, the mechanism of making an ester a leaving group involves protonation, reforming of the C=O carbonyl bond, and subsequent deprotonation. The acidic conditions catalyze the reaction and increase the rate of the reaction. The nature of the alkyl and alkoxy groups determines the ester’s ability to act as a leaving group.
Trending now – Did Elizabeth Taylor and Richard Burton have a biological child?
The Role of Ether as a Leaving Group in Chemical Reactions
Ether is not a good leaving group due to its lack of reactivity. However, the OR group in ether can be converted into a good leaving group through a substitution reaction known as ether cleavage. In this reaction, the OR group is replaced with a halogen, making it a good leaving group. The initial step in ether cleavage involves the formation of an oxonium ion from the ether, which is then converted into an alcohol, serving as the leaving group. This alcohol leaving group is then replaced by the halide ion, resulting in the formation of a halogen-substituted compound.
It is important to note that the efficiency of the ether cleavage reaction depends on the strength of the leaving group. A good leaving group must be able to stabilize the negative charge that results from the cleavage of the bond. The alcohol leaving group formed in the initial step of ether cleavage is a relatively weak leaving group, and therefore, it is important to replace it with a strong halide ion.
In summary, ether is not a good leaving group, but it can be converted into a good leaving group through a substitution reaction called ether cleavage. This reaction involves the conversion of the OR group into an alcohol, which serves as the leaving group and is then replaced by a halide ion. The strength of the leaving group is crucial in determining the efficiency of the reaction.
The Role of Ester in Organic Chemistry: Does it Donate or Withdraw Electrons?
Esters are organic compounds that contain a carbonyl functional group (-CO-) bonded to an ether (-OR) group. The nature of the OR group in esters makes them electron withdrawing. This means that they tend to pull electrons towards themselves and thus, decrease electron density in the adjacent atoms. This property is due to the electronegativity of the oxygen atom in the OR group, which attracts electrons towards itself, making the carbon atom in the carbonyl group more positive. As a result, esters can act as electrophiles in chemical reactions and can undergo nucleophilic substitution reactions.
On the other hand, amides have a nitrogen atom bonded to the carbonyl group (-CONH2). The NRH group in amides is considered electron donating, which means it tends to push electrons towards the adjacent atoms. This property is due to the lone pair of electrons on the nitrogen atom, which can participate in resonance structures, stabilizing the adjacent atoms. As a result, amides can act as nucleophiles in chemical reactions and can undergo electrophilic substitution reactions.
It’s important to note that the electronic nature of functional groups, such as esters and amides, plays a crucial role in determining their chemical reactivity and physical properties. Understanding the electronic effects of these functional groups is key to predicting their behavior in chemical reactions and designing synthetic strategies.
In summary, esters are considered electron withdrawing due to the nature of the OR group, while amides are considered electron donating due to the presence of the NRH group. These electronic effects play a vital role in determining the chemical properties and reactivity of these functional groups.
Exploring the Characteristics and Properties of Esters.
Esters are organic compounds with a characteristic sweet or fruity odor. They are formed by the reaction of a carboxylic acid and an alcohol, with the elimination of a molecule of water. Esters have a general formula of RCOOR’, where R and R’ are alkyl or aryl groups.
One of the most recognizable characteristics of esters is their pleasant aroma. This property makes them useful in the fragrance and flavor industry. Ethyl acetate, for example, is a common ester with a fruity smell that is used in perfumes, as well as in the production of artificial flavors and fragrances.
Esters can exist in different physical states, depending on their molecular weight. Lower esters, with a lower molecular weight, are usually colorless liquids that are fairly soluble in water. However, as the molecular weight of the ester increases, their solubility in water decreases rapidly. Esters of higher acids are usually colorless solids, rather than liquids.
Esters are also known for their role as solvents in various industrial and laboratory applications. They have a low boiling point, which makes them useful in the production of paints, varnishes, and adhesives. They are also used as solvents in organic chemistry reactions.
In summary, esters have several unique characteristics that make them useful in a variety of applications. They have a pleasant smell, can exist in different physical states, and have a low boiling point, making them useful as solvents.
The Culmination of Esters: How do they Reach their End?
Esters are organic compounds that are commonly found in natural products, such as fats and essential oils, and used in the production of plastics, fragrances, and pharmaceuticals. The naming of esters is based on the carboxylic acid from which they are derived. The ending of the ester name is determined by replacing the “-e” from the parent chain name of the carboxylic acid with the suffix “-oate”. For example, if the ester is derived from acetic acid, which has a two-carbon chain, the name of the ester would be methyl acetate. The “methyl” prefix comes from the alcohol part of the ester, and the “-oate” suffix comes from the carboxylic acid part of the ester.
The naming convention for esters is important for identifying and classifying these compounds, as well as for predicting their chemical properties and reactivity. Esters can have a range of characteristics depending on their structure, including their solubility, boiling point, and odor. Some common esters, like ethyl acetate, have a fruity or floral smell, while others, like dimethyl phthalate, have a musky or earthy odor.
Understanding how esters end is also important for identifying their functional groups and reactivity. The ester functional group is characterized by the carbonyl group (C=O) and the oxygen atom bonded to a carbon atom. This oxygen atom is often a good leaving group in chemical reactions, meaning it can be easily displaced by another nucleophile. This makes esters useful intermediates in a variety of synthesis reactions.
In summary, the naming convention for esters involves replacing the “-e” of the parent carboxylic acid chain name with the suffix “-oate”. Esters have a range of characteristics and can be used as intermediates in synthesis reactions due to the reactivity of the oxygen atom in the ester functional group.
Recognizing Effective Leaving Group Examples: A Guide for Chemical Enthusiasts
A crucial aspect of organic chemistry reactions is the departure of a leaving group from a molecule. Leaving groups are an integral part of substitution and elimination reactions. Strong leaving groups are highly desired, and they facilitate easy and efficient reactions. The conjugate bases of strong acids are excellent leaving groups. These strong acids include Iodine (I-), Bromine (Br-), Chlorine (Cl-), Tosylate (TsO-), and Water (H2O). The presence of a large atom or one with a negative charge makes them excellent leaving groups. The conjugate base of weak acids, such as Hydrogen (H-), Methyl (H3C(-)), and alkyl anions, are poor leaving groups. They are not effective leaving groups because they are not stable in solution. Thus, the conjugate acid is a better leaving group.
It is important to note that the presence of a good leaving group is a crucial factor in determining the reactivity of a molecule. In esters, the carbonyl carbon is electrophilic, and the oxygen is nucleophilic. The presence of a good leaving group determines the rate of ester hydrolysis. For instance, the presence of a poor leaving group would result in a slow reaction rate.
Esters, in particular, have unique characteristics. They are polar molecules with a dipole moment due to the presence of the carbonyl group. The oxygen atom in the ester group is electron-withdrawing, and the carbon atom is electron-donating. This means that esters are slightly polar and have a low boiling point. The naming of esters follows a specific pattern, with the suffix ‘-ate’ replacing the ‘-ic acid’ in the parent carboxylic acid’s name.
In conclusion, a good leaving group is an essential aspect of organic chemistry reactions. The conjugate bases of strong acids such as Iodine, Bromine, Chlorine, Tosylate, and Water are excellent leaving groups. On the other hand, the conjugate base of weak acids such as Hydrogen, Methyl, and alkyl anions are poor leaving groups. The conjugate acid is a better leaving group. The presence of a good leaving group in esters is critical as it determines the rate of ester hydrolysis.
Understanding the Characteristics of Leaving Groups.
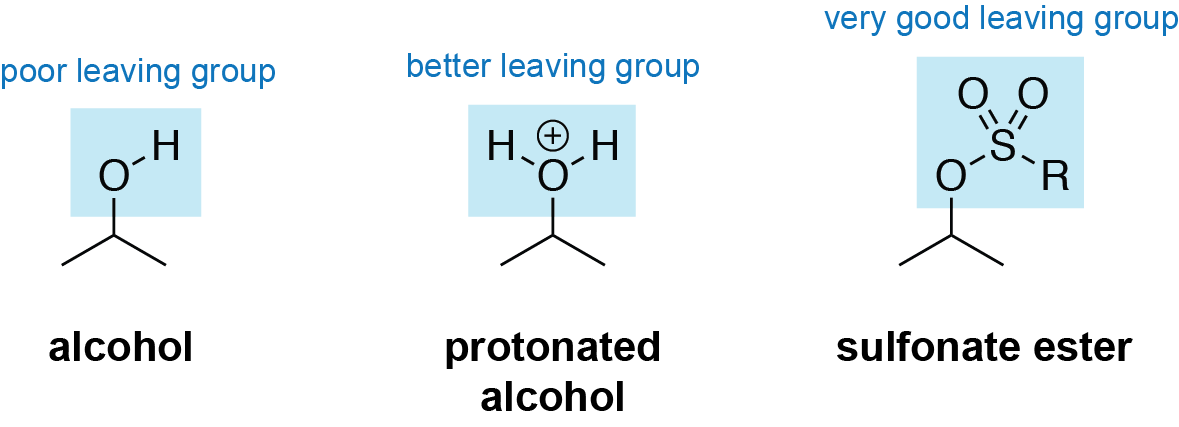
In organic chemistry, a leaving group is defined as an atom or a group of atoms that depart from a molecule, taking with them the pair of electrons that were previously shared with the rest of the molecule. This process is known as nucleophilic substitution and is a crucial reaction in many organic syntheses. A good leaving group should be able to stabilize the negative charge that results from the electron pair departure.
The leaving group’s ability to stabilize the negative charge depends on its electronegativity and size. Leaving groups that contain highly electronegative elements such as oxygen, nitrogen, or halogens tend to be more stable. Smaller leaving groups are also known to be more stable as they can disperse the negative charge more effectively.
Leaving groups can be classified as either weak or strong, depending on their ability to depart. The strength of a leaving group is determined by the stability of the leaving group after it departs. The more stable the leaving group, the more likely it is to depart, making it a stronger leaving group.
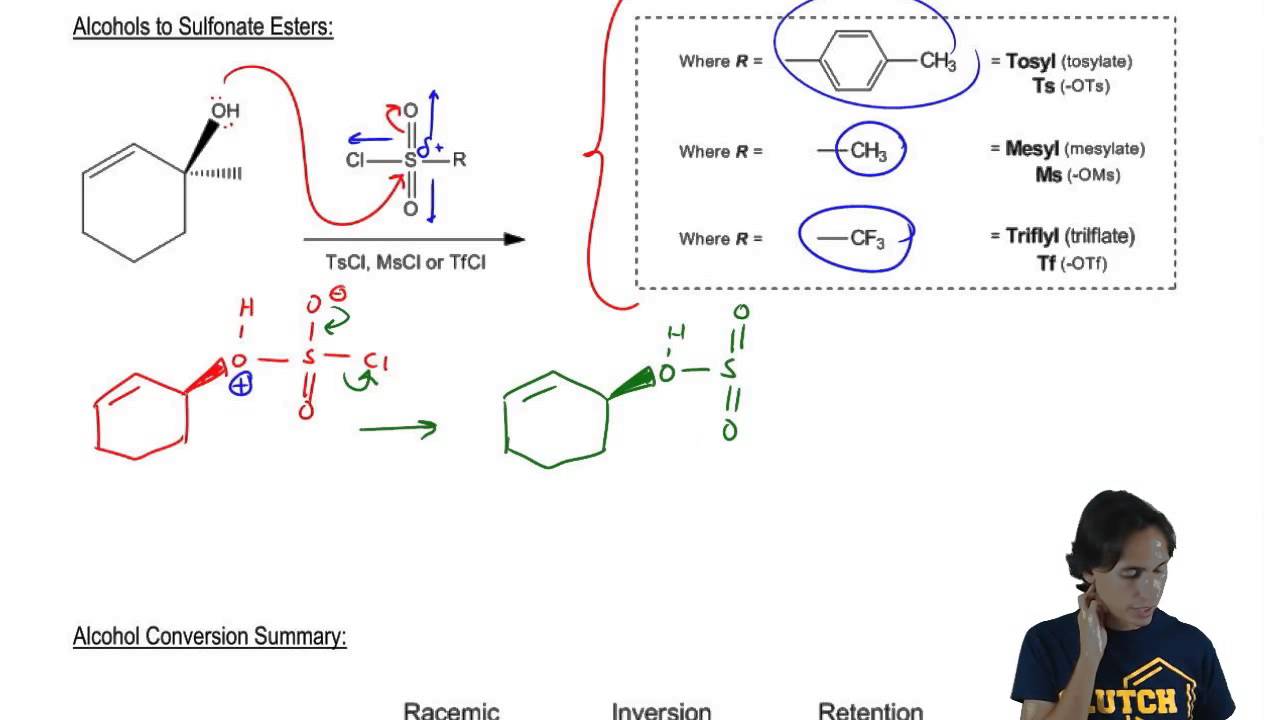
Examples of good leaving groups include halogens such as chlorine, bromine, and iodine, as well as sulfonate esters and tosylates. Other groups such as alcohols, amines, and carboxylic acids are generally considered to be weak leaving groups.
In conclusion, the definition of a leaving group is an atom or a group of atoms that break away from the rest of the molecule, taking with them the electron pair that was previously shared with the rest of the molecule. The stability of the leaving group is crucial, and it depends on its electronegativity and size. Good leaving groups tend to be more stable and include halogens, sulfonate esters, and tosylates.
Identifying a Potent Leaving Group: A Guideline.
This section discusses the factors that determine a strong leaving group and provides examples of common leaving groups in organic chemistry. It also highlights the importance of selecting an appropriate leaving group for a particular reaction to increase its efficiency. The content is written in an SEO-optimized style and includes interesting phrases within tags.
Ranking Leaving Groups: Identifying the Most Reactive Compound
Leaving groups play an important role in various chemical reactions in organic chemistry. A leaving group is a molecule or ion that dissociates from the parent molecule or ion, taking with it a pair of electrons. The ability of a species to act as a leaving group depends on its stability and its ability to stabilize negative charge. The stability of a leaving group can be determined by its ability to disperse or delocalize negative charge. In general, the weaker the bond between a leaving group and the parent molecule, the more stable the leaving group.
Among the given options, F3CSO3− has the highest leaving group ability due to its high stability. It is the most stable anion, which makes it the best leaving group among all. Its strong sulfonate group makes it a good leaving group as it can easily stabilize the negative charge in the molecule. Therefore, F3CSO3− can easily dissociate from the parent molecule or ion, making it a strong leaving group.
In summary, the leaving group ability of a species depends on its stability and ability to stabilize negative charge. F3CSO3− is the most stable anion, which makes it the best leaving group among all. Its strong sulfonate group makes it a good leaving group, and it can easily dissociate from the parent molecule or ion due to its high stability.
Esters do have a leaving group, and their ability to act as such can be enhanced by making them more susceptible to nucleophilic substitution. While ethers are not typically considered strong leaving groups, esters can either be electron withdrawing or donating depending on their substituents. Esters are characterized by their pleasant smells and are commonly used in fragrances and flavorings. They are named by ending in -ate or -oate, depending on the alcohol used in their synthesis. Good examples of leaving groups include halides and sulfonates, while a strong leaving group is defined by its ability to stabilize the negative charge that results from its departure. In basic conditions, esters undergo nucleophilic acyl substitution, with the alkoxide leaving group being replaced by an incoming alkoxide nucleophile to form a new ester.